This is an in vitro diagnostic medical device that helps diagnose kidney disease by quantifying the concentration of Cystatin-C contained in human serum and plasma (EDTA, Heparin) using latex turbidimetric immunoassay.
■ Test Procedure for Analyzers (Hitachi 7180)
- Assay Mode: 2 Point End 18-28
- Wave length(main/sub): 570/800nm
■ Open reagent
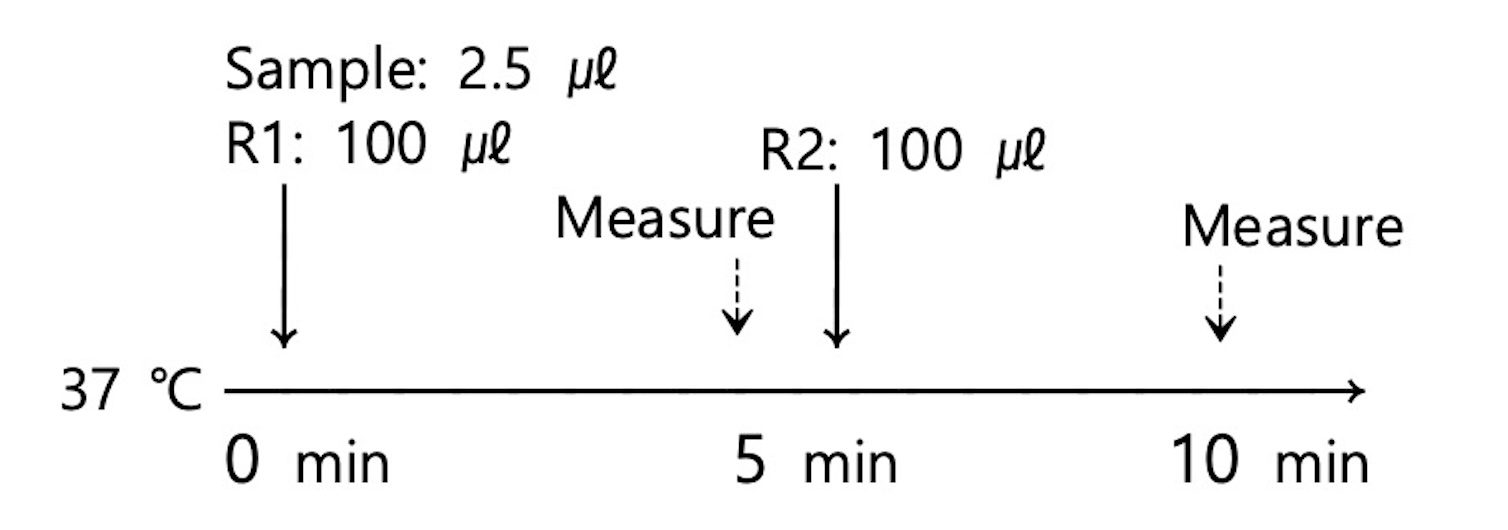
■ Linearity: 0.1~8.5 mg/L
■ Sensitivity: Initial absorbance 0.8-1.5 / Reagent Blank(AAbs): 0.01 or less
■ Accuracy: Within 100±10% of the expected value when measuring standard substances of base activity
■ Reproducibility: CV less than 10% when measuring 0.85 mg/L Cystatin-C sample 10 times.
■ Interference reaction:
Bilirubin : 20 mg/dL
Hemoglobin : 500 mg/dL
Intralipid : 5 %
EDTA 2Na : 200 mg/dL
Heparin sodium : 40 U/mL
Sodium fluoride : 2000 mg/dL
Sodium citrate : 1000 mg/dL